Sound familiar? Here’s why: if your system only helps you accept applications, you don’t have a research management system—you have an inbox with better branding.
If you’ve been fighting the same battle or thinking it might be time to get out of the rut, this article is for you. We’re unpacking what a research management system should be doing for you, and why collecting applications is just one small piece of the puzzle.
Let’s talk about the messy middle: what happens after funding is approved, and why solving it takes more than just another intake form.
Key takeaways
- Many research management systems only cover applications, but post-award is where real complexity and operational risks arise.
- Fragmented systems lead to stalled approvals, difficult reporting, compliance gaps, and increased administrative burden.
- A modern system should automate workflows, provide full lifecycle visibility, simplify reporting and compliance, and offer a single collaborative platform.
What gets lost when your system only manages applications
On paper, the application is the beginning. But for most research offices, it’s also the point where your system stops doing its job.
And it’s not because your team is disorganised or lacking skill. It’s because many research systems—including internally built or custom solutions—were designed with a narrow definition of success: submissions. But if you’ve managed post-award workflows, you know that’s not how it works. The real complexity—approvals, variations, reporting, compliance—kicks in the moment funding is approved.
And when your system isn’t built to handle those moments, it’s not just frustrating—it’s operationally risky.
Here’s what starts to slip through the cracks when your research management system only manages applications.
Approvals that stall and disappear
Approvals should be the simplest part of your post-award process. A disbursement request is raised, the right person reviews it, and the system records the decision. But that’s rarely how it plays out.
Instead, approvals go missing in someone’s inbox. Or they happen verbally. Or they’re signed off in a PDF that gets uploaded to the wrong folder, or not uploaded at all. You’re left wondering whether something was approved or just assumed to be.
And while that might be manageable for one small grant, it becomes a serious problem when you’re managing multiple funding streams, variations, or ethics requirements across an extensive portfolio.
The root issue? Most systems don’t enforce structured approval pathways. There’s no visibility, no timestamp, no audit trail—just emails, manual follow-ups, and best guesses.
Here’s what that looks like in practice:
- A project lead submits a milestone variation via email. No one notices for two weeks because the approver was away, and there was no system alert to escalate it.
- A budget amendment is verbally approved in a meeting, but the formal record never makes it back into the system.
- You’re trying to verify a disbursement for audit purposes, but can’t confirm who signed off—or when—because the decision happened outside the platform.
- Alternatively, you’re using software to manage one or more of these steps, but it’s not integrated, and information is siloed.
These aren’t process hiccups, but systemic gaps.
When approvals stall or disappear, they not only delay progress but also increase financial and compliance risk. And they chip away at your team’s confidence in the system they’re meant to rely on.
A research management system that can’t handle multi-stage, role-based approvals—with clear ownership and traceability—puts your entire post-award workflow on shaky ground.
Reporting that takes weeks, not minutes
You shouldn’t need a detective, a spreadsheet artist, and two weeks’ notice just to answer a routine reporting request. Yet that’s precisely what it feels like when your data lives across five tools and none of them talk to each other.
Essentially, you end up piecing together spending, milestones, outcomes, and approvals from email threads, PDF uploads, shared folders, and your team’s collective memory.
Take a typical scenario: A funder requests a mid-term impact report. You open the system, only to realise it doesn’t track progress. So you search for budget updates in Excel, check ethics milestones in an email chain, pull variation approvals from SharePoint, and manually format everything into a funder-friendly narrative.
What should be a three-click task becomes a cross-platform scavenger hunt. And that’s assuming you’ve got a full team. If a key staff member is on leave or no longer with the organisation, the data gaps multiply.
These aren’t just time costs—they’re risk multipliers. Incomplete data can lead to late reporting, missed obligations, and frustration among funders. Worse, it erodes your credibility. When you can’t answer questions with confidence—or consistency—it signals you’re not in control.
A modern research management system should offer real-time visibility across your grant portfolio. That means standardised fields, embedded reporting templates, automated dashboards, and built-in audit trails. If you’re still assembling reports from scratch, the system isn’t working—you are.
Compliance that’s patchy at best
Compliance in research isn’t a “nice to have”—it’s table stakes. Funders expect clear documentation. Ethics committees require complete visibility. Audit teams want trails, not stories. And yet, in many organisations, compliance still lives in a patchwork of folders, emails, and institutional memory.
When your research management system doesn’t track compliance requirements—when it can’t flag missing documents, escalate overdue milestones, or log actions automatically—you’re left building a safety net out of sticky notes and calendar reminders.
And the cracks start to show. This table illustrates just a few ways compliance issues arise when your system isn’t functioning properly.
| Situation | What goes wrong |
|---|---|
| A compliance deliverable is tied to a variation, not the original agreement | It gets missed entirely because no one tracked it separately |
| An ethics condition is documented but not uploaded | The evidence either disappears or lives on someone’s desktop, inaccessible to the team |
| A funder asks how a decision was made | There’s no clear record of who approved what, or when |
These moments may seem insignificant in isolation. But in a tightly regulated research environment, they compound fast—and the consequences can be delays, funding risk and loss of institutional credibility.
What’s needed is a system that doesn’t just log compliance manually, but actively supports it—something most custom-built tools struggle to maintain as programs scale or policies evolve. It’s not about ticking boxes but building trust with your funders, your researchers, and your internal stakeholders.
Because when compliance lives outside the system, risk lives everywhere.
➔ Read more about how to manage compliance in grant and research management
The hidden costs of fragmented systems
We’ve covered the operational risks—missed approvals, delayed reports, compliance gaps. But underneath all of that is a quieter cost: the strain it places on your team.
When the system stops short, people compensate. They chase updates manually. Recreate reports from scratch. Rely on memory instead of visibility. Over time, that workaround culture isn’t just inefficient—it’s exhausting. Here’s what it looks like:
Collaboration that breaks down at every stage
Research operations touch every corner of an organisation—project leads, finance, legal, ethics, external partners. But without a shared system to anchor those moving parts, collaboration quickly turns into confusion.
Approvals get emailed to the wrong person. Budget updates fall out of sync. Milestones are marked complete in one place but missing in another. And while they may seem like one-off mistakes, they aren’t. This is what happens when the system can’t hold the complexity your team deals with every day. The more stakeholders involved, the more exposed the gaps become.
A strong research management platform doesn’t just store information—it connects the dots between people, roles, and decisions. And when that connection is missing, even the most capable teams start slipping.
Admin burden that grows quietly, then explodes
Most research admin teams don’t fall apart overnight. They stretch, absorb and find workarounds. Until one day, the spreadsheet crashes, someone goes on leave, or a major reporting deadline hits—and everything spills over.
That’s what fragmented systems do best: they mask the load until it’s too much to carry.
You see it in the daily friction:
- Hours spent cross-checking data between systems
- Manual updates to status trackers no one fully trusts
- New staff needing weeks to learn “how we do things here”
What starts as “just how we work” becomes a quiet drain on time, focus, and team morale. And if you’re relying on a homegrown system, that burden only grows—because every workflow change or reporting need becomes another internal ticket.
So, what should a modern research management system really do?
By now, the challenges are clear, and if you’re managing complex grants across multiple years, funding bodies, and reporting lines, you know that patching over the gaps just doesn’t cut it.
What you need is sound infrastructure, a system that’s deeply embedded in your processes, gives your team full visibility, and adapts to the way you actually work—not the other way around.
Here’s what that looks like in practice. And this is exactly what we’ve built at Enquire—because we’ve seen what’s at stake when the right system isn’t in place.
Automate workflows for approvals, variations, and disbursements
You shouldn’t be chasing email threads to see if something’s been signed off. With Enquire, you can set up workflows that mirror your internal processes—routing requests to the right people, escalating when needed, and capturing every decision along the way.
Get real-time visibility across your entire grant lifecycle
Whether you’re managing three grants or three hundred, Enquire gives you a clear view of everything in motion—from intake to close-out. Drill down by program, funding body, team, or risk level and know exactly where things stand without toggling between tools.
Generate reports and audit trails in just a few clicks
If reporting still feels like a time sink, your system isn’t doing enough. Enquire captures what you need as you work, so when it’s time to report, everything’s already there. You can export funder-ready data, view real-time dashboards, and hand over clean audit trails without a last-minute scramble.
Build compliance into the way you work
We don’t believe in bolted-on compliance. With Enquire, it’s baked in—at every stage. From ethics milestones to financial documentation, our workflows help your team stay on track, avoid surprises, and meet obligations by default.
Give your team (and your stakeholders) one place to work
When files are in one system, approvals in another, and communication buried in inboxes—collaboration stalls.
Enquire gives you and your stakeholders a shared source of truth. Internal teams can stay aligned. External users can check status, submit docs, and respond to requests through secure portals—without relying on back-and-forth emails.
Adapt as your research program grows
Your organisation isn’t static and your system shouldn’t be either. With Enquire, you can configure workflows, fields, approval chains, and documentation requirements to match your structure. Whether you’re dealing with government-funded programs or community-led initiatives, we make it easy to scale without reengineering your entire process.
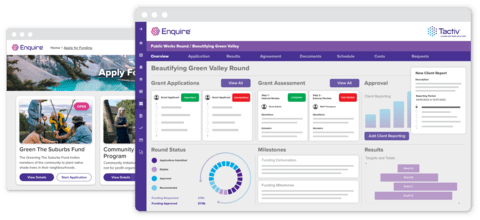
Enquire as a Research Management System
How the Heart Foundation uses Enquire to manage their research program
When the Heart Foundation first approached us, they were managing a high-volume, multi-year research program using a legacy system that couldn’t keep up. Program management had become fragmented across multiple platforms, introducing inefficiencies, increasing admin load, and adding compliance risk.
With Enquire, they were able to streamline the full grant lifecycle into one platform. The result?
- 100+ configurable workflows set up to support scholarships, large-scale research initiatives, and patient-impact projects
- 35 grants awarded from 140+ applications in 2024 alone
- Centralised reporting and real-time data visibility, allowing staff to track progress, measure impact, and make funding decisions backed by evidence
- Improved governance and ethics adherence, with automated workflows that reduce risk and bias across assessments
And with over $2M in funding distributed through Enquire to support life-saving heart research, the Foundation now has the infrastructure to scale impact without scaling complexity.
➔ Read the full Heart Foundation case study here
And if your team is navigating similar complexity—fragmented tools, growing compliance demands, or simply too much manual work—we’d love to show you how Enquire can help. Book a demo and see how other research organisations are simplifying the grant lifecycle—from first application to final acquittal.